INTRODUCTION
Aflatoxins (AFs) are among the most potent toxigenic and carcinogenic secondary fungal metabolites produced by members of the fungal genus Aspergillus1. These naturally occurring group 1 carcinogens2 often contaminate a wide variety of food commodities including dietary staples and animal feed. There are four distinct types of aflatoxins: aflatoxins B1 (AFB1), aflatoxins B2 (AFB2), aflatoxins G1 (AFG1), and aflatoxins G2 (AFG2). Among them, AFB1 is the most toxic and abundantly producing mycotoxin3. Its hydroxylated metabolite aflatoxin M1 (AFM1) is produced after AFB1 detoxification2. AFM1 is also well-known for its toxic effects and appears in several animal products like milk, meat, and eggs1. The liver is responsible for metabolizing the Afs, therefore it is the most targeted organ2. AFs exert a toxic effect through DNA damage and mutations leading to oxidative damage4. These low molecular weight potent mutagens and teratogens impact health differently on acute and chronic exposure5. Hence, these toxins are of greatest concern and threat to food safety, and public and veterinary health. Their effect causes significant economic losses by reducing the quality of food, increasing disease burdens, and reducing productivity measures2. Acute effects cause severe cellular damage, pulmonary oedema, liver necrosis, and even death with a case fatality rate of 40%6. Chronic exposure may range from a series of effects like carcinogenicity to multiple organs, especially the liver and kidneys, and immune system dysfunction, adverse pregnancy and birth outcomes, malnutrition, and growth retardation in children5,7. Moreover, risk of hepatocellular carcinoma (HCC) increased due to the synergistic interaction between aflatoxins and hepatitis virus7.
The effect of AFs on several hematobiochemical parameters has also been studied, it is varied in animals and humans. Numerous experimental studies on cattle, pigs, rats, and rabbits reported varying levels of effect on hematobiochemical parameters4. In animals, AFB1 exposure causes the lysis of erythrocytes and disturbs iron absorption5. Correspondingly, in pregnant females in China, it was the cause of anemia7. It also disturbed liver and kidney function tests in study participants from Malaysia and Egypt6,8. The tolerable consumption levels of aflatoxins for humans have not been set yet, but it is a fact that any level of AFs is not safe for human consumption1. Infants and young children ingest more food than adults, therefore intake of toxin may be higher on a kilogram body weight basis. Thus, children are among the more vulnerable population due to their greater risk of consumption, higher metabolic rate, and lower detoxification capabilities9,10. In Pakistan, studies reported AFs contamination levels above the tolerable limits in many staple foods like rice, wheat, maize, lentils, milk, and dairy products, indicating a breach in food safety law enforcement2. Therefore, the high occurrence of AFs in food is a serious public health concern in Pakistan. However, it is still an unheeded issue, and inadequate data are available on its effect on health. Certain countries have AFs control regulations firmly enforced in food and feed10. But in Pakistan, uniform proper laws are unavailable and not enforced strictly for AFs control.
Changing climatic conditions, particularly temperature and humidity can significantly influence the toxicity and occurrence of AFs. High temperatures (>30℃) and humidity levels (>70%) promote fungal growth on standing crops, especially during food transportation and storage. Hence, the population is at immense risk of AFs exposure if the situation is not controlled10.
The AFB1-lysine adduct and its metabolite AFM1 in urine can be used as molecular biomarkers to assess the extent of exposure in the human population2. AFB1 indicates direct dietary exposure of past up to 3 months while AFM1 indicates recent 1–3 days exposure of AFM11,11. Hence, both are reliable biomarkers for epidemiological investigations and have been used in several studies7,9. The current research is part of a cross-sectional study targeted at evaluating the exposure levels of aflatoxins and related effects on growth in children1. We hypothesized that exposure to AFs and their metabolites through food and environment has adversely affected several hematobiochemical parameters in children. Additionally, we used the margin of exposure (MoE) approach to assess the risk of liver cancer from AFB1 exposure in children.
METHODS
Sampling
This cross-sectional design sampled 238 children attending the outpatient department (OPD) or the nutrition clinic of the Children’s Hospital and The Institute of Child Health between January and September 2020. A structured questionnaire was administered to collect sociodemographic data of the study participants (Supplementary file).
Ethical declaration
This study was approved by the Institutional Review Board (IRB) of The Children’s Hospital and The Institute of Child Health (approval no. 59525, dated; 28-10-2019) and the Institutional Review Committee for Biomedical Research (IRCBR), University of Veterinary and Animal Sciences (approval no. 033/IRC/BMR, dated; 04-02-2019), Lahore, following the ethical principles Declaration of Helsinki (DoH/Oct 2008) by the World Medical Association. Guardians of all children gave informed consent for voluntary participation. Data was anonymized and confidential.
Sample collection
A trained phlebotomist collected 238 blood samples following the standardized procedure in dipotassium (K2) EDTA (ethylenediamine tetra-acetic acid) and clot activator vials. The hospital pathology laboratory analyzed these samples for hematobiochemical parameters. After analysis, serum samples were separated into 1 mL Eppendorf tubes. Parents/guardians of the children were guided on urine sample collection by the investigator. Sterile plastic urine containers/pediatric urine bags were used to collect urine samples. The urine and serum samples were then sent to the laboratory of the Department of Epidemiology and Public Health, University of Veterinary and Animal Sciences (UVAS), Lahore, under cold chain (2–8℃) conditions. At UVAS, urine samples were analyzed weekly for AFM1 using ELISA. Serum samples were stored at -80℃ for 1 year to subsequent AFB1-lysine adduct analysis. Serum samples were transported to the University of Georgia, Athens, USA, maintaining a cold chain (2–8℃). This adduct is known for its stability at -80℃ over the years, i.e. 15 years of storage caused about 6% degradation, and at ambient temperature ensuring reliable results12,13.
Quantitation of hematobiochemical parameters
Hematology auto-analyzer Sysmex XP 100 (Sysmex Corporation, Japan) was used for measuring hemoglobin (Hb), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), red blood cell counts (RBCs), white blood cell counts (WBCs), platelet counts (PLT) and differential counts using ethylenediaminetetraacetic acid (EDTA) blood. The serum was obtained by centrifuging blood at 3000g for 15 min (Kubota Centrifuge Model 2810, Tokyo, Japan) and AU480 (Beckman coulter, USA) chemistry auto-analyzer was used for measuring the following biochemical parameters: total bilirubin (TB), direct bilirubin (DB), alkaline phosphatase (ALP), alanine transaminase (ALT), aspartate transaminase (AST), gamma-glutamyl transferase (GGT), total protein (TP), albumin (Alb), urea, and creatinine. Immunochromatographic tests (Zhejiang Orient Gene Biotech Co. Ltd Zhejiang, China) were used to qualitatively detect Hepatitis B Surface Antigen (HBsAg) and Hepatitis C virus antibody (HCV) in serum.
Quantitation of AFM1
Following the manufacturer’s instructions, an ELISA kit (Helica Biosystem, Inc., Santa Ana, CA, USA) was utilized to quantify AFM1 in the urine samples of study participants. It is a direct ELISA in which a high-affinity AFM1 antibody coated microwell polystyrene plate was used. To summarize the procedure, all the reagents, standards and samples were equilibrated to room temperature. Samples were centrifuged (Centrifuge 5702, Eppendorf Biotech business Hamburg, Germany) at 3000g for 5 min and the supernatant was diluted first with distilled water (1:20) and then with the assay buffer (1:2); 100 μL of the diluted supernatant was added to the antibody coated microwell plate and incubated for 1 h. After washing 100 μL of conjugate was added and incubated for another 15 min. Then the following washing 100 μL of substrate was added and incubated for further 15 min. Finally, the reaction was stopped by adding 100 μL of stop solution. The absorbance of the plates was read at 450 nm filter on an ELISA reader (PR 4100 Absorbance Microplate Reader, California, United States). The standard curve was plotted using stabilized urine standards (0, 0.15, 0.8, 1.5, and 0.4 ng/mL) and had a correlation coefficient of 0.96. The limit of detection (LOD) evaluated for the method was 0.015 ng/mL and final AFM1 concentrations were normalized to creatinine prior to statistical analysis. The mean recovery of 0.544 ng/mL and 1.98 ng/mL spiked urine samples was 96.4% (range: 78–111%) and 96.5% (range: 73–109%), respectively, as per the manufacturer.
Quantitation of serum AFB1- lysine adduct
For exposure of AFB1 in participants, serum AFB1-lysine adduct was measured by a newly developed HPLC fluorescence method validated by Qian, and the method was described in detail elsewhere10. Briefly, serum samples were thawed and heated to inactivate suspected human pathogens. Further samples were digested with pronase (ratio 1: 4) at 37°C for 3 h in a water bath (Precision, Lab Mechanics, Winchester, Virginia). The AFB1-lysine digest was separated and purified using an Oasis Waters Max (1CC 30 mg) filter solid-phase extraction cartridge (Milford, MA, USA). Before loading the digested samples, the cartridge was pre-primed with methanol (MeOH) and equilibrated with water, then the sample was sequentially washed with water, MeOH, and ammonium hydroxide. The flow rate was 1 mL/min. AFB1-lysine was eluted by formic acid and vacuum dried concentration (Labcono Centrivap concentrator Kansas City, MO, USA). After those contents were reconstituted in MeOH and analysis was performed on Agilent 1260 HPLC-fluorescence system (Sant Clara, CA, USA). The mobile phase was composed of a linear gradient profile of monobasic buffer A (pH 7.2), ammonium phosphate (20 mM), and buffer B MeOH (100%). Agilent C18 column (particle size 5 μm, 250×4.6 mm) was used for chromatographic separation and adduct concentration was analyzed by fluorescence at maximum excitation (405 nm) and emission wavelength (470 nm). To ensure quality assurance and quality control, one standard and two quality control samples were also run in each analysis lot. A standard calibration peak was generated through elution of AFB1-lysine standard with a retention period of approximately 13.0 min. Our method’s detection limit (LOD) was 0.4 pg/mg. The final concentration of AFB1-lysine adducts was normalized to serum albumin.
Risk assessment
The daily dietary AFB1 intake was estimated by urinary biomonitoring and the following equation was used to assess the probable daily intake (PDI in ng/kg bw/day) of AFB1:
where C is the biomarker concentration normalized to creatinine (ng/mg of creatinine), V is the daily urine excretion (mL) and daily urine volume assumed to be 700 to 1500 mL/day for age group 1–11 years, W is the body weight (kg), and E is the excretion rate (%), based on Zhu et al.3 and the AFB1 excretion rate was estimated to be 2%. The margin of exposure (MoE) was used to evaluate the risk of AFB1 exposure, as AFB1 is a genotoxic carcinogen. The MoE can be computed as:
where the BMDL10 was set at 400 ng/kg bw/day by the European Food Safety Authority (EFSA) for aflatoxin.
Statistical analysis
R software version 4.0.4 and R Studio version 1.4.1106 were used for statistical analyses. Hematobiochemical parameters had a positive skew and non-normal distribution. Frequency distribution and descriptive statistics were used to present and calculate the mean, median, and interquartile range. A robust generalized linear model with a gamma family distribution was used to evaluate the impact of dietary AFB1 and AFM1 on blood parameters. We fitted multiple models in R software using the function glmrob with a log link function and with mqle (mixed quantile regression) method to examine the relationship between response and explanatory variables. Explanatory variables were adjusted for age, sex, area of residence, mother’s education level, family monthly income, participation of the child in physical activities, vaccination status of the child, frequency of illness, and nutritional status of the child, i.e. wasting, stunting and underweight.
RESULTS
Characteristics of study participants
The median age of the 238 participants (42.4% females, 57.6% males) was 3.0 years (IQR: 2.0– 4.5). Anemia was prevalent in 65.13% (155/238) of the children. RBC counts were higher than normal in 14.3% (34/238) and lower than normal in 8.4% (20/238) of the participants. WBC counts were high in 4.6% (11/238) and low in 2.1% (5/238). Total protein was high in 10.9% (26/238) and low in 8.4% (20/238) of the participants, while albumin was high in 1.9% (4/238) and low in 0.84% (2/238). Liver enzymes were elevated, 5.0% (12/238) for ALT, 42% (99/238) for AST, 7.0% (14/198) for GGT, and 22.0% (51/232) for ALP. Kidney function tests were normal for most participants except one with high urea, two with high creatinine, and three with low creatinine levels. Sociodemographic characteristics and hematobiochemical parameters are presented in Table 1. No participants had a HBV infection, while 0.84% (2/238) of the participants were infected with HCV.
Table 1
Sociodemographic and hematobiochemical characteristics of children visiting the hospital, January–September 2020, Lahore (N=238)
Correlation between serum AFB1-lysine adduct urinary AFM1 levels
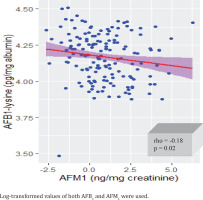
Urinary AFM1 was detected in 65.5% (156/238) of the participants. The urinary AFM1 median level was 1.9 ng/mg creatinine (IQR: 0.82–6.0) while non creatinine adjusted was 0.57 ng/mL (IQR: 0.23–1.41). All (238/238) serum samples had AFB1-lysine adduct with a median level of 10.66 pg/mg albumin (IQR: 6.25–20.32) and a range of 0.72–255.63 pg/mg albumin (Table 2). A statistically significant weak association (p=0.02, rho= -0.18) was found between blood AFB1-lysine adduct and excreted AFM1 in urine of the participants (Figure 1).
Table 2
Aflatoxin B1 (AFB1) albumin adduct, aflatoxin M1 (AFM1), probable daily intake (PDI) and margin of exposure (MoE) of AFB1 in children visiting the hospital, January–September 2020, Lahore (N=238)
Probable daily intake (PDI) and margin of exposure (MoE)
The PDI of AFB1 was calculated for participants with detectable urinary AFM1 levels (65.5%, 156/238). We found 91% (142/156) of them had a PDI of more than 1 ng/kg bw/day. The median PDI was 8.16 ng/kg bw/day (IQR: 3.18–26.82) and there were no significant differences in PDI by sex, age, and residence. The median MoE was 49.0 (IQR: 93.65–159.77) (Table 2). This indicates a high risk of liver cancer from AFB1 exposure in children, as EFSA considers an MOE ≥10000 to be of low concern for public health.
Effect of AFM1 on hematobiochemical parameters
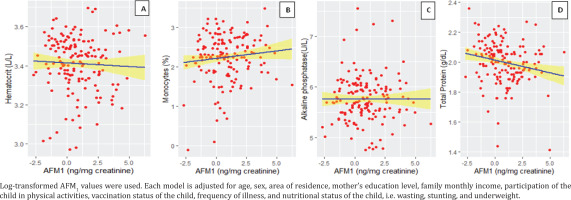
The results of regression analysis indicated that HCT, monocytes, ALP and TP levels were influenced by AFM1. When all other factors remained constant, HCT increased by 0.0011 (p=0.048) with changes in AFM1 from negative to positive. The estimated coefficient for monocytes, ALP, and TP showed that for every unit increase in AFM1, the expected value of monocytes decreased by 0.027 (p<0.001), the expected value of ALP decreased by 4.12×104 (p=0.01) and the expected value of TP decreased by 0.0048 (p=0.042) (Table 3).
Table 3
Generalized linear regression models of the impact of serum AFB1-lysine adduct and urinary AFM1 levels on hematobiochemical parameters in children visiting the hospital, January–September 2020, Lahore (N=238)
Effect of AFB1 on hematobiochemical parameters
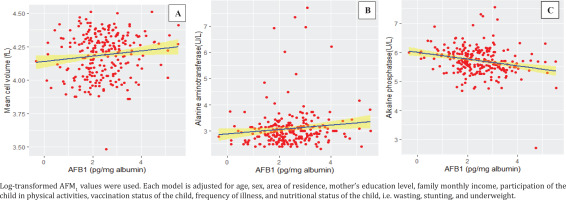
The regression analysis revealed that when other variables held constant the presence of AFB1 had a significant effect on hematobiochemical parameters. The estimated coefficient demonstrated that with every unit increase in AFB1, the expected value of MCV decreased by 9.88×106 (p=0.018) and the expected value ALT decreased by 1.92×104 (p=0.014), while the expected value of ALP increased by 6.66×106 (p=0.028) (Table 3).
DISCUSSION
In the current study, AFM1 was detected in 65.5% of participants and the median level of AFM1 was 1.9 ng/mg when adjusted for creatinine and 0.57 ng/mL when not adjusted. Similar studies from Pakistan reported non-creatinine-adjusted AFM1 levels ranging from 0.023 ± 0.048 ng/mL to 1.86 ± 0.25 ng/mL1,4. All the participants had AFB1 lysine adduct levels in their blood with median level of 10.66 pg/mg albumin. No previous studies from Pakistan reported blood AFB1 levels. Also, our study found higher blood AFB1 levels than those reported by studies from our neighboring countries, Bangladesh and Nepal14,15. This difference could be the lack of uniform and properly enforced laws regarding aflatoxin contamination throughout the country. Additionally, Pakistan is among the top ten countries affected by global warming and a high warm and humid climate promotes the growth of toxigenic fungi.
The PDI from urinary AFM1 was calculated to estimate the amount of AFB1 ingested from food. The median PDI was 8.16 ng/kg bw/day in our study participants and 91% of them had a PDI >1 ng/kg bw/day. Our PDI is higher than those reported in Chile, Brazil and Europe that ranged from 0.09 to 3.25 ng/kg bw/day3,16,17. The MoE derived from PDI was 49.0, which is far below the safe threshold of 10000 recommended by the European Food Safety Authority (EFSA). This indicates a high risk of aflatoxin exposure, which is both carcinogenic and toxigenic. A similar study from Pakistan also found a low MoE of 13.2 in their participants18. There are no safe levels of aflatoxin intake, so it is important to reduce the contamination as much as possible. Infants and children are especially vulnerable to aflatoxin exposure and its effects, as they have higher intake relative to their body weight19.
The most affected organ due to aflatoxin toxicity is liver because aflatoxins are primarily metabolized by the liver. AFs not only cause degeneration and necrosis of the liver, but they also lead to bile duct proliferation and infiltration of inflammatory cells. Primarily, they cause high expression of death receptor pathway and apoptosis of hepatocytes through an extrinsic mechanism, so they have direct contribution to develop hepatocellular carcinoma (HCC)2. Worldwide, about 4.6–28.2% of HCC cases are attributable to the AFs exposure, and it is the sixth most common cancer globally20. Additionally, presence of hepatitis virus in the body might have a synergistic effect that could increase the chances of HCC by 30 times when interacted with HBV and 5.8 times when interacted with HCV20,21. HBV is endemic in Pakistan, and it stands second in global burden of HCV infection. WHO has declared HCV a public health threat in Pakistan by 203021. Some studies have reported the prevalence of viral hepatitis (HBV and HCV) in children from Pakistan (Table 4). In our study, we also found two positive cases of HCV infection (2/238) in children along with a remarkably high prevalence of AFB1 (100%) and AFM1 (65.5%). This could pose a serious risk for liver problems in these children.
Table 4
Prevalence of hepatitis in children reported by different studies in Pakistan
The toxic effects of AFs exposure have the capability to alter liver enzymes4. We found that AFs exposure had a significant impact on ALT and ALP levels in our study participants. Our results are different from a study from Pakistan where no association was reported between AFs and liver enzymes ALT and ALP in children22. We found that AFB1 had a negative effect on ALT in the regression model (coefficient= -0.0000192, p=0.014) when holding other factors constant, which may indicate that AFB1 causes liver dysfunction and damage by triggering the death of hepatocytes through a death receptor pathway23. One other study from Pakistan reported an association between AFs and ALT levels in their study participants but did not specify positive or negative effects4. Similarly, ALT levels were found to be significantly higher in treatment groups including placebo group in an experimental study of calcium montmorillonite clay in children and in an observational study with comparison groups, where one group was exposed to organic dust8,24. ALP levels are variable in aflatoxicosis. In our regression analysis, ALP levels were decreased with AFM1 levels and increased with AFB1 exposure. It may be due to AFB1 detoxified by the liver enzyme cytochrome P450, which leads to increased production of toxic metabolites including aflatoxicol, AFB1-8, 9-epoxide, AFM1, and aflatoxin P14. Some metabolites such as AFB1-8, 9-epoxide are highly reactive and can induce oxidative stress and liver injury. ALP is an enzyme involved in the detoxification process, and its activity may increase as a compensatory response to counteract AFB1-induced liver damage. The early stages of aflatoxin-induced liver damage may involve hepatocyte injury (elevated ALT), followed by compensatory mechanisms (increased ALP) as the liver repairs itself.
On the other hand, AFM1 may not have the same detoxification pathway or may not induce ALP activity to the same extent, leading to a negative association between AFM1 and ALP levels. AFB1 exposure can trigger an immune response, leading to inflammation and oxidative stress in the liver25. Increased ALP levels may reflect the activation of immune cells involved in the inflammatory response. AFM1 may not elicit the same immune response. One observational baker study and experimental studies revealed that ALP levels increased with exposure to AFB126,27. The altered activity of liver enzymes in serum is an indication of hepatic damage. The activities of hepatic enzymes decrease as metabolic processes take out the hepatic enzymes from serum. The timing of hepatic enzymes determinations in terms of pathogenesis of aflatoxicosis is important and enzymatic activity must be interpreted in the context of the temporal aspect of aflatoxicosis28.
Total proteins levels were negatively associated with AFM1. Some experimental studies reported similar negative association where AFs exposure decreased total proteins25,29. However, a cross sectional study in Ghana reported that high AFs levels were significantly associated with high serum proteins in adults30. AFM1 may affect total proteins by altering the expression of enzymes involved in amino acid metabolism, such as proline dehydrogenase (PRODH), which catalyzes the oxidation of proline to glutamate31.
Monocytes play key role in innate immunity5. We found that the level of AFM1 was negatively associated with monocytes in the blood. A similar result was reported by an experimental study that showed decrease in monocyte count due to AFs exposure32. On the other hand, a study in children who were given calcium montmorillonite clay (UPSN), a substance that can reduce bioavailability of AFB1 found their monocytes levels increased24. This suggests that AFs may impair the function of monocytes and weaken innate immunity.
We found a high prevalence (65.13%) of anemic children in our study population, it was not associated with AFs exposure. In a recent study from Pakistan, researchers found similar results22. Moreover, experimental studies from China and Ghana reported that AFs exposure caused maternal anemia7,33. However, the magnitude of damage caused by AFs extremely depends on the dose, exposure period and route of the toxin and may range from acute to chronic problems. We found that with AFs exposure HCT increased and MCV decreased, in contrast to a study that reported an increase in MCV29. The liver plays a crucial role in the production and regulation of blood cells including red blood cells. Thus, aflatoxin exposure may affect the liver’s ability to produce or maintain the proper balance of red blood cells. Additionally, aflatoxin exposure can lead to oxidative stress and inflammation in the body. These factors can affect the function and life span of red blood cells, potentially resulting in their size and proportion in the blood.
Limitations
This study has some limitations. We did not document the complete dietary intake of children to evaluate the possible sources of aflatoxin exposure, including the possibility of contaminated air from a potentially contaminated food source not being evaluated. Our questionnaire lacks specific quantitative estimates at an individual level, and we did not conduct food analysis for aflatoxin contamination. It is important to note that contamination levels can change based on food preparation methods (i.e. ready meals vs homemade). Furthermore, we did not examine the same children over time to detect the effect of aflatoxin on hematobiochemical parameters over a period of time. From our analysis, we can conclude that both toxins had impact on hematobiochemical parameters.
To address these limitations, we recommend designing and conducting longitudinal and cohort studies with larger sample sizes that repeatedly examine the same individuals to detect any changes that might occur over time. Additionally, food samples should be analyzed for the parent aflatoxin/metabolite from various sources, to calculate receptive concentrations. Such studies are scarce and can provide valuable information about the health status of the population, especially children.
CONCLUSIONS
Our study provides valuable insights into hematobiochemical parameters influenced by AFB1 and AFM1 exposure. Significantly altered liver and hematological parameters with AFs exposure may indicate AFs as potential toxicants in children and a risk to public health. The high prevalence and concentrations of AFs in children demand an effective implementation plan to control AFs contamination in food. The prevalence of hepatitis C in the studied children along with aflatoxin exposure may deteriorate their health and alarmingly increase the burden on the health system. Further, case-control studies are recommended to see the role of AFs in the development of various types of cancers, particularly associated with the liver, in our population.