INTRODUCTION
Ketamine, a versatile anesthetic agent with the IUPAC name 2-(2-chlorophenyl)-2-(methylamino)cyclohexan-1-one, has been a significant player in medical and recreational spheres since its synthesis in 1962 by Calvin Stevens at Parke-Davis1. Initially developed as a safer alternative to phencyclidine (PCP), ketamine quickly gained recognition for its effectiveness in both human and veterinary medicine1,2. Ketamine is a noncompetitive NMDAR antagonist and primarily targets glutamate, the central nervous system’s main excitatory neurotransmitter. Ketamine’s distinctive dissociative action and partial agonism of opioid mu-receptors contribute to its ability to provide consistent sedation, analgesia, and amnesia during painful procedures while maintaining laryngeal reflexes and ensuring respiratory and cardiovascular stability2,3. More recently, ketamine has shown promise in treating conditions such as treatment-resistant depression, bipolar disorder, anxiety, and chronic pain1,2.
However, the same properties that make ketamine valuable therapeutically also contribute to its potential for abuse. Recreational users, attracted by its dissociative effects, including intense hallucinations and out-of-body experiences, often refer to as ‘special K’ or ‘super-K’1. Ketamine’s rapid onset (30 seconds IV, 5–30 minutes intranasally, 20 minutes orally), effects lasting up to three hours with an elimination half-life of 2–4 hours, and affordability, make it a popular choice for recreational use2,4. Additionally, curiosity and peer influence contribute in its widespread appeal5. Recreationally, ketamine is commonly inhaled as a powder, with usage patterns varying significantly. Some individuals consume more than 1 g per session and use it on consecutive days1.
Reports of ketamine abuse have been documented since the late 1960s and have spread globally since the 1970s and 1980s, expanding from North America to Europe and Asia1. A study evaluating US National Poison Control data found an 81.1% increase in ketamine exposures among individuals aged ≥13 years between 2019 and 20215. Most of these cases involved males (57.1%), with the primary reasons being intentional misuse or abuse (39.5%), suspected suicides (19.7%), and accidental exposures (18.9%)6. In the United Kingdom, the Crime Survey of England and Wales (CSEW) reported a significant rise in ketamine usage among those aged 16–24 years from 0.8% in 2006–2007 to 2.9% in 2018–20195. In the past decade, Hong Kong and China have also reported widespread ketamine abuse despite its controlled substance status1.
While ketamine is generally safe and fatalities solely from its use are rare, abuse of the drug poses significant health risks and often requires medical intervention for severe psychological effects1,5. Higher doses, particularly when combined with other substances like alcohol, can lead to severe outcomes, including fatalities2,6. A 2023 systematic review highlighted that ketamine was implicated in 79% of overdose cases and 89.1% of deaths related to co-ingestants, emphasizing the dangers of polysubstance use7.
Chronic, unsupervised ketamine use is strongly associated with urogenital toxicity, including acute kidney injury, dysuria, and hematuria7. Additionally, ketamine undergoes extensive metabolism in the liver via the cytochrome P450 system (CYP 3A4), and prolonged use can lead to significant liver damage, presenting as abdominal pain, jaundice, and pruritus8. The mechanisms underlying these toxic effects are not fully understood but are believed to involve direct damage to the urinary and biliary tract linings by high concentrations of ketamine metabolites, particularly norketamine9. At the molecular level, ketamine and norketamine induce mitochondrial dysfunction, endoplasmic reticulum stress, and calcium-regulated ERK1/2 activation, contributing to bladder damage10.
Despite the growing body of research on ketamine’s adverse effects, there remains a notable gap in the literature regarding its potential hematological complications. A comprehensive review of PubMed using keywords such as ‘ketamine’, ‘ketamine retroperitoneal hematoma’, ‘ketamine hematological complications’, and ‘adverse effects’, yielded no reported cases of severe hematological complications or retroperitoneal hematoma associated with illicit ketamine use. This gap is critical given the rising prevalence of ketamine abuse and its potential to cause severe health outcomes.
We present a case of ketamine-induced coagulopathy resulting in severe anemia and a large retroperitoneal hematoma. By documenting this case, we aim to contribute to the current body of knowledge and raise awareness about the potentially life-threatening hematological consequences of chronic ketamine abuse.
CASE PRESENTATION
The patient was a 40-year-old male who presented to the emergency department at Staten Island University Hospital with a three-week history of weakness and lethargy, accompanied by gross hematuria with clots for five days. His medical history was significant for chronic intranasal ketamine misuse spanning approximately 11 years, with no history of intravenous drug use. The patient’s ketamine usage had peaked at 3 g per day over the first 10 years before reducing to 500 mg per day. In the 10th year, he attempted to discontinue use, and by the 11th year, his usage became mostly intermittent. His last ketamine use was one week prior to presentation. The patient’s medical history also included chronic interstitial cystitis with intermittent hematuria and chronic kidney disease (CKD) stage 3A, characterized by a glomerular filtration rate (GFR) between 45 and 59 mL/min/1.73 m2. Notably, the patient had experienced an episode of MRSA bacteremia 16 months prior, which was treated with intravenous daptomycin. Following this episode, the patient underwent significant weight changes, losing 60 pounds after the bacteremia and then gaining 15 pounds in the month leading up to the current presentation.
Upon admission, the patient exhibited tachycardia with a heart rate of 122 bpm and a blood pressure of 118/60 mmHg. Laboratory measurements (Table 1) revealed severe anemia with a hemoglobin level of 3.8 g/dL, an elevated white blood cell count of 21 K/μL, and hypovolemic hyponatremia with a sodium level of 123 mmol/L. His creatinine was significantly elevated at 4.6 mg/dL, compared to a baseline of 1.3 mg/dL. Urine analysis showed a large leukocyte esterase concentration and elevated WBCs at 120/HPF. Urine and serum toxicology screens were positive only for oxycodone. Imaging studies revealed right basilar haziness on chest X-ray, bilateral retroperitoneal hematomas within the posterior pararenal spaces (Figure 1), asymmetrical enlargement of the left psoas and iliopsoas muscles (Figure 2), and bilateral renal enlargement with indeterminate renal lesions on abdominal computed tomography (CT) scan.
Table 1
Patient’s laboratory measurements at initial emergency department presentation (arrows represent deviation from normal reference range)
| Measurements | Patient values |
|---|---|
| WBC (K/μL) | 21.65 ↑ |
| Hgb (g/dL) | 3.8 ↓↓ |
| Na+ (mmol/L) | 123 ↓ |
| Creatinine (mg/dL) | 4.6 ↑↑ |
| PT (s) | 12.30 |
| INR | 1.12 |
| Fibrinogen assay (mg/dL) | 573 ↑ |
| D-dimer quantitative (ng/mL DDU) | 2361 ↑ |
Figure 1
Computed tomography of the abdomen without contrast showing large bilateral retroperitoneal hematomas in the posterior pararenal spaces
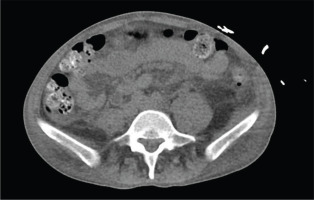
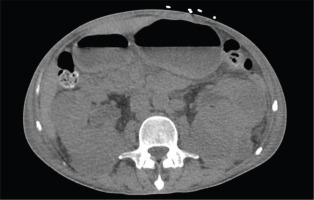
Figure 2
Computed tomography of the pelvis without contrast revealing asymmetric enlargement of the left psoas and iliopsoas muscle, suggestive of intramuscular collection/hematoma
Further investigations were conducted to fully assess the patient’s condition. Blood cultures returned positive for MRSA. A coagulation profile showed a plasma prothrombin time (PT) of 12.30 s, an International Normalized Ratio (INR) of 1.08, a fibrinogen assay of 573 mg/dL, and a Dimer Quantitative Assay measured 2361 ng/mL D-dimer units (DDU). Iron studies indicated a combination of iron deficiency and anemia of chronic disease. The patient also showed elevated C-reactive protein (CRP), positive antinuclear antibodies (ANA), and positive proteinase 3 (PR3) anti-neutrophil cytoplasmic antibodies (ANCA).
The plan included blood transfusion of eight units of packed red blood cells to raise hemoglobin levels above 7 g/dL. Antibiotic therapy was started with initial empiric IV vancomycin, later switched to IV daptomycin upon MRSA confirmation, with the addition of IV ceftaroline due to persistently positive cultures. AKI was managed through fluid management and monitoring of renal function. For hematuria management, the urology team recommended an indwelling Foley catheter, which the patient declined.
The nephrology service attributed the AKI superimposed on CKD to factors such as poor solute intake or low antidiuretic hormone levels. Of note, a kidney biopsy was considered but not performed given the patient’s compromised renal function and ongoing antibiotic regimen.
The patient’s hemoglobin levels showed a steady improvement throughout his hospital course and renal function stabilized. Once the patient’s blood cultures returned negative for five consecutive days, he was discharged home with a peripherally inserted central catheter (PICC) line for intravenous daptomycin. Upon discharge, the patient received instructions to follow up with nephrology, urology, rheumatology, hematology/oncology, and infectious disease specialists for ongoing management of his complex medical condition. The patient’s total hospital stay was 22 days.
DISCUSSION
This case illustrates a complex clinical scenario of severe anemia, retroperitoneal hematoma, and hematuria in a patient with chronic ketamine abuse, highlighting the potential for severe hematological complications associated with long-term illicit drug use. The patient’s 11-year history of intranasal ketamine use is a crucial factor in evaluating the etiology of the retroperitoneal hematoma, particularly given the well-documented adverse effects of chronic ketamine abuse on renal function. Retroperitoneal hematoma as a consequence of ketamine abuse has not been previously reported, thus challenging our current understanding of the complications associated with chronic ketamine use.
Chronic ketamine abuse has been associated with various urological and renal complications, including interstitial cystitis, hydronephrosis, and renal dysfunction. Huang et al.8 reported that chronic ketamine use resulted in diffuse bladder wall thickening (88.9%), reduced bladder volume (66.7%), and bladder inflammation (44.4%). Retroperitoneal hematomas are typically associated with anticoagulant therapy, trauma, or certain vascular pathologies10. Their occurrence in the context of drug abuse, especially ketamine, is not well-documented.
While MRSA bacteremia was present, several factors argue against it as the primary cause of retroperitoneal hematoma in this patient. Retroperitoneal hematomas are rarely associated with MRSA bacteremia alone, especially in the absence of other risk factors such as anticoagulant therapy or trauma11. Additionally, the patient’s coagulation profile showed normal results, which is atypical for sepsis-induced coagulopathy (SIC). SIC is characterized by significant alterations in coagulation parameters, typically presenting with abnormalities in several key coagulation markers, including prolonged prothrombin time (PT) and decreased platelet counts, which are crucial components of the diagnostic criteria11.
In contrast, chronic ketamine abuse is linked to significant vascular damage, which may contribute to hematoma formation without altering systemic coagulation parameters10. Prolonged ketamine exposure can lead to endothelial dysfunction, crucial for vascular health maintenance10. Moreover, chronic ketamine users exhibit reduced levels of peripheral blood serum vascular endothelial growth factor (VEGF) protein12. As VEGF is essential for maintaining the integrity of existing blood vessels, promoting repair when damaged, and stimulating growth of new blood vessels, reduced VEGF levels can theoretically increase the risk of hematoma by leaving blood vessels susceptible to damage13.
The patient exhibited elevated inflammatory markers such as CRP, ANA, and PR3-ANCA, which are typically associated with vasculitis. However, the etiology in this case is more likely to be drug-induced rather than primary vasculitis. While ANCA-associated vasculitis (AAV) related to ketamine use is not well-documented in the literature, drug-induced autoantibodies, including ANCA, have been reported with substances like cocaine, which is commonly adulterated in street ketamine5,14. In this case, the patient’s symptoms predominantly align with ketamine’s known urological and renal effects, rather than typical vasculitic manifestations. The absence of a personal or family history of autoimmune diseases further supports a drug-induced etiology. Drug-induced AAV often presents with atypical features and may lack the full clinical spectrum of primary AAV15. The transient nature of drug-induced autoantibodies and the long-term immunological effects of chronic ketamine abuse require further investigation.
Limitations
As a case report, our study has inherent limitations. The findings are based on a single patient, which limits the generalizability of our conclusions. Additionally, while ketamine is often the primary component in street samples, analysis from 2013 to 2020 revealed that these samples frequently contain adulterants, including amphetamines, 3,4-Methyl enedioxy methamphetamine (MDMA), cocaine, and synthetic cathinones5. This complicates the attribution of observed effects solely to ketamine. Further research, including case series and controlled studies, is necessary to elucidate the impact of ketamine on hematological sequelae and to validate these findings in a broader population. Such studies will enhance our understanding of the potential adverse effects and help establish more comprehensive clinical guidelines.
CONCLUSION
While ketamine is acknowledged for its psychomimetic effects and potential for abuse, its role in precipitating hematological complications, as witnessed in our case, remains a scarcely documented phenomenon. Physicians should maintain a heightened index of suspicion for toxicity in patients with a history of substance abuse present with profound anemia or unexplained bleeding. Lastly, this case should prompt an increased emphasis on patient education about the potential dangers of illicit drug use, including their unanticipated hematological sequelae.


