INTRODUCTION
A detailed understanding of the carcinogenic potential of environmental chemicals is one of the critical issues on public health. The mechanisms of chemical carcinogenesis are traditionally divided into two stages, initiation and promotion, known as two-stage carcinogenesis1, while currently, multistage carcinogenesis is widely accepted2. Lately, the two-stage carcinogenesis tests in the various organs3, medium-term multi-organ carcinogenesis tests4, and other short-term carcinogenicity tests5 have been developed and utilized. In addition, the test using transgenic animals to detect tissue-specific genetic mutations was adopted as an OECD test guideline, OECD TG 488; Transgenic Rodent Somatic and Germ Cell Gene Mutation Assays6, and multi-endpoint genotoxicity assay to use gpt delta transgenic rats7 is used as an in vivo outstanding assay of genotoxic evaluation. These tests will not only contribute to the refinement and rapidity of carcinogenicity assessment but will also be useful to elucidate the organ specificity of initiation and promotion effects. It is expected that the combination of these tests will deepen our understanding of mechanisms and target organs for carcinogenesis.
It is well-known that genotoxic carcinogens exhibit initiation effects on the target organs of carcinogenesis, and in the medium-term multi-organ carcinogenicity study, several initiators are used to induce selective initiation in the various target organs8. Recently, however, it was reported the treatment of genotoxic carcinogens induced gene mutations in non-target organs of carcinogenesis. Hakura et al.9 showed that benzo(a)pyrene (BaP) induces mutations in the colon and the small intestines in mice, which are not recognized as target organs of carcinogenesis. Thus, the evidence may suggest not only initiation effects but also promotion effects of the test chemicals are possible to occur in the organs other than the targets of carcinogenesis. Less is known about non-genotoxic carcinogens with promotion effects, so hazard identification and evaluation are more difficult to detect.
Non-genotoxic carcinogens are known to induce tumors through their long-term exposure in rodents10. The contamination of tumor promoters in foods, and its carcinogenic risk have been investigated and discussed11. Since there is concern that promotion effects to organs other than the target organs, may enhance the effects of genotoxic environmental substances that are simultaneously exposed, it is possible promotion effects to occur in organs other than those in which tumor formation was noted in the 2-year carcinogenicity studies. The effects of food contaminants on the gastrointestinal (GI) organs are considered an important point for public health since the contaminants are directly exposed to GI organs, especially if the contaminants in foods contained substances with tumor promotion effects. For analysis of in vivo tissue-specific promotion effects, assays that detect cell proliferation such as immunohistochemical staining for BrdU12-14, PCNA13, and Ki6715, and RDS assay16 are commonly utilized. Although tumor promotion studies on digestive systems with initiation have been conducted in several chemicals including foods17, cell proliferation assays would be useful to avoid the non-genotoxic effects of initiation substances and to estimate actual toxicological effects under realistic exposure conditions in humans. Thus, verifying the absence of significant cell proliferation effects on GI organs under subacute exposure to non-genotoxic substances may provide an important perspective for substantial risk assessment of non-genotoxic carcinogens.
The purpose of this study is to evaluate cell proliferative activity in organs of the gastrointestinal tract after oral exposure to a carcinogenic agent that targets organs other than the gastrointestinal tract for carcinogenesis, by animal experiments. Thus, we conduct a 4-week study to confirm the effects of tumor promoters on GI organs to mimic accidental exposure of tumor promoters as contaminants in foods. TPA and PB were treated orally in drinking water for 4 weeks in male rats, and cell proliferation was examined by histopathology and immunohistochemical BrdU labeling indices.
METHODS
Study design
As non-GI-organ-targeted carcinogenic promoters, TPA (target organ: skin) and PB (target organ: liver) were selected as test compounds, a 4-week repeated dose study in Crl:CD(SD) rats was conducted. Rats were given PB (100; 300; and 900 μg/mL) or TPA (0.5; 1.5; and 4.5 μg/mL) orally in drinking water for 28 days. Histopathological examination and BrdU immunostaining were conducted to examine the cell proliferation activity in the target and GI organs. Then, histopathological findings and the number of proliferative cells detected were compared to the control group to determine the adverse effect of the compounds in GI organs.
Animals
Four-week-old male Crl:CD(SD) rats (SPF animals) were purchased from Charles River Laboratories, Japan Inc (Shiga, Japan), and acclimated for 8 days before allocation. Rats were individually housed in clear plastic cages (W 257 × D 426 × H 200 mm) with soft chip bedding (Hara Shouten, Co., Ltd., Japan) in an animal facility with a temperature of 22 ± 3ºC, a humidity of 55 ± 15%, ventilation frequency of at least 10 times/hour, and a 12-hour light/dark cycle (7 am – 7 pm). MF pellet diet (Oriental Yeast Co., Ltd., Tokyo, Japan) and tap water were available ad libitum. Rats were allocated into 7 groups (5 males/group) based on randomized body weights one day before the commencement of the treatment, and the treatment was commenced at the age of 5 weeks.
The study was conducted in accordance with the Law for the Humane Treatment and Management of Animals (Law No. 46, May 2014), Standards Relating to the Care and Management of Laboratory Animals and Relief of Pain (Notice No. 84 of the Ministry of Environment dated September 2013), Guidelines for Proper Conduct of Animal Experiments (Science Council of Japan, June 2006), ‘Basic policies for the conduct of animal experiment in academic research institutions’ (Notice No. 02201 of the Ministry of Health, Labor and Welfare, February 2015), Guidelines for Proper Conduct of Animal Experiments (Science Council of Japan, June 2006). This study was approved by the animal experiment committee of Kao Corporation (Approval number: F16041-0000; Date: 15 March 2016), and DIMS Institute of Medical Science Inc., (Approval number: 16205; Date: 4 July 2016).
Materials
The 12-O-tetradecanoylphorbol-13-acetate (TPA) (synonym: Phorbol 12-myristate 13-acetate, purity: 100%) and bromodeoxyuridine (BrdU) were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Phenobarbital sodium salt (PB) (synonym: 5-Ethyl-5-phenylbarbituric acid sodium, purity: 93.4%) was purchased from Tokyo Chemical Industry Co., LTD. (Tokyo, Japan). Dimethyl sulfoxide (DMSO) was purchased from Wako Pure Chemical Industries, Ltd. (Tokyo, Japan). Physiological saline was purchased from Otsuka Pharmaceutical Factory, Inc. (Tokyo, Japan).
Preparation of TPA and PB treatment solutions
For TPA, 25 mg of TPA was mixed with DMSO, and 5 mg/mL of TPA stock solution was prepared. Aliquots of 540 μL of TPA stock solution were divided into tight glass bottles and stored in a carcinogen storage freezer. Before treatment, TPA stock solution (540 μL) was thawed at room temperature, and added to a DMSO using a micropipette, and made primary treatment solution, as 0.5 and 1.5 mg/mL. Each primary solution was diluted 1000 times with tap water, and given at doses of 0.5, 1.5, and 4.5 μg/mL. For PB, 100, 300, and 900 mg of PB were dissolved in 1000 mL of tap water and prepared treatment solutions at the concentrations of 100, 300, and 900 µg/mL.
Dose setting
Based on the results of a 2-week dose range finding study (Doses of TPA: 0.15, 0.5, and 1.5 µg/mL in the drinking water, Doses of PB: 125, 250, and 500 µg/mL in the drinking water), the doses in the present study were selected at 0.5, 1.5 and 4.5 µg/mL for TPA, and 100, 300 and 900 µg/mL for PB. The study design is shown in Table 1. The rats were given TPA and PB orally in drinking water for 28 days. Animals in Group 1 freely accessed tap water during the treatment period.
Table 1
Dose group design in a 4-week repeated dose study
| Group | Test material | Dose level (μg/mL)a | Number of rats |
|---|---|---|---|
| 1 | - | 0 | 5 |
| 2 | TPA | 0.5 | 5 |
| 3 | TPA | 1.5 | 5 |
| 4 | TPA | 4.5 | 5 |
| 5 | PB | 100 | 5 |
| 6 | PB | 300 | 5 |
| 7 | PB | 900 | 5 |
Observations and examinations
All animals were examined daily for general conditions, including mortality and clinical signs. Body weights were measured using an electronic balance (LA4200, Sartorius K.K.) at the start of the experiment, weekly during the treatment period, and before the necropsy. Food consumption per cage was measured weekly, and water consumption per cage was measured daily. Test material intakes were calculated based on the daily water consumption per animal.
On the day of necropsy, 1% (w/v) of BrdU solution dissolved in the physiological saline was administered to the rats once intraperitoneally. One hour after BrdU administration the animals were euthanized by bleeding from the abdominal aorta under isoflurane anesthesia and subjected to necropsy based on guidelines listed in the Animal section (Materials and Methods section). Heart, lungs, liver, kidneys, spleen, gastrointestinal tracts including tongue, esophagus, stomach, duodenum, jejunum, ileum, cecum, colon, rectum, and skin (back) were excised and fixed in 10% buffered formalin solution. The above organs/tissues were trimmed, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (HE), and then examined histopathologically. In addition to the routine histopathological examination, to investigate the proliferative activity, the above organs, and tissues were subjected to immunohistochemistry for BrdU (Monoclonal Mouse Anti-Bromodeoxyuridine, Dako, Glostrup, Denmark) by Avidine-Biotine-peroxidase Complex method. The BrdU-labeled cells in a total of 1000 cells in each stained slide were counted and calculated as the BrdU labeling index (LI, %).
Statistical analysis
The significance of differences between the control and treated groups for each parameter was analyzed and evaluated at p<0.05 or p<0.01. The data of body weight, food consumption, water consumption, and BrdU LI were assessed using Bartlett’s test (evaluated at p<0.05). If the data were homogeneous in Bartlett’s test, the data were analyzed using parametric Dunnett’s multiple comparison test (two-sided); if not, they were analyzed with non-parametric Steel’s test (two-sided). The significance of intergroup differences in incidences of findings from gross pathology and histopathology were analyzed by the one-sided Fisher’s exact probability test. The two-sided Wilcoxon test was employed for the comparison of graded changes. The statistical analysis was not performed for the data of general conditions. The statistical analyses were performed using Stat Light 2000 (Yukms Co., Ltd.,).
RESULTS
Survival and general condition
No deaths and no abnormalities in general condition were observed in all groups, including the control and treated groups of TPA and PB during the treatment period (data not shown).
Body weight, food, and water consumption, and test-materials intake
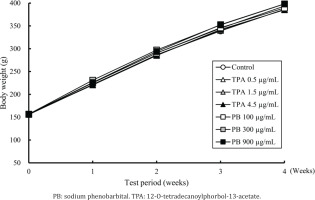
During the treatment period, there were no toxicologically remarkable changes in body weights (Figure 1) and food consumption (Table 2) noted in the treated groups of TPA and PB compared with the control group. In water consumption, although transient but significant decrease or tend to decrease was found in the PB 900 µg/mL group at the beginning of the treatment compared to the control group, toxicologically significant changes were not noted both in TPA and PB treated groups during the treatment period (Table 3). Based on the data on water consumption, average intakes of TPA were estimated to be 64.0, 158.0, and 448.3 µg/kg/day in the 0.5, 1.5, and 4.5 µg/mL groups, respectively. Average intakes of PB were also estimated to be 11104.2, 37123.6, and 90624.1 µg/kg/day in the 100, 300, and 900 µg/mL groups, respectively (Table 4).
Table 2
Food consumption (g/animal/day) in a 4-week repeated dose study
Table 3
Water consumptiona (g/animal/day) in a 4-week repeated dose study
| Group | Test material | Dose level (μg/mL) | Days | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0–1 | 6–7 | 13–14 | 27–28 | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| 1 | - | 0 | 27.83 | 2.47 | 30.17 | 3.25 | 34.67 | 3.69 | 34.33 | 5.01 |
| 2 | TPA | 0.5 | 31.00 | 3.00 | 32.83 | 5.01 | 38.17 | 5.53 | 37.33 | 4.01 |
| 3 | 1.5 | 24.00 | 2.65 | 27.83 | 2.02 | 31.67 | 0.76 | 32.33 | 3.06 | |
| 4 | 4.5 | 24.00 | 2.65 | 26.50 | 1.32 | 28.67 | 2.57 | 31.00 | 3.28 | |
| 5 | PB | 100 | 27.17 | 2.36 | 29.00 | 2.50 | 33.33 | 5.03 | 32.50 | 3.50 |
| 6 | 300 | 31.33 | 1.61 | 30.17 | 3.75 | 35.67 | 5.69 | 36.83 | 5.35 | |
| 7 | 900 | 17.50** | 3.04 | 25.17 | 1.04 | 28.00 | 1.80 | 30.83 | 6.17 | |
Table 4
Dose levels, total intakes, and average intake of test materials (two carcinogenic promoters) in a 4-week repeated dose study
| Group | Test material | Dose level (μg/mL) | Total intakea (μg/animal) | Average intakeb | |
|---|---|---|---|---|---|
| (μg/animal/day) | (μg/kg/day) | ||||
| 1 | - | 0 | 0.0 | 0.0 | 0.0 |
| 2 | TPA | 0.5 | 498.1 | 17.8 | 64.0 |
| 3 | 1.5 | 1247.8 | 44.6 | 158.0 | |
| 4 | 4.5 | 3489.8 | 124.6 | 448.3 | |
| 5 | PB | 100 | 87516.7 | 3125.6 | 11104.2 |
| 6 | 300 | 298450.0 | 10658.9 | 37123.6 | |
| 7 | 900 | 724500.0 | 25875.0 | 90624.1 | |
Postmortem examination
Gross pathology
At necropsy, no macroscopic changes were found in the TPA and PB treated groups.
Histopathology
The histopathological findings are summarized in Table 5. In the histopathological examination, only treatment-related change was observed in the liver in PB groups. Hepatocellular hypertrophy was found in all PB-treated groups and the grade of the finding was dose-related and the incidence of the finding was increased with statistical significance. Histopathological lesions in the other organs/tissues observed in the PB-treated groups and any organ/tissue examined in the TPA-treated groups were considered to be sporadic since these were commonly found in the control animals in the test facility.
Table 5
Histopathological findings in a 4-week repeated dose study
| Group | Control | TPA | PB | ||||
|---|---|---|---|---|---|---|---|
| Dose level (µg/mL) | 0 | 0.5 | 1.5 | 4.5 | 100 | 300 | 900 |
| Total number of rats | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Findingsa (number of rats) | |||||||
| Heart | |||||||
| Normal | 5 | 4 | 5 | 4 | 3 | 5 | 4 |
| Mononuclear cell infiltrate/fibrosis, myocardium (1) | 0 | 1 | 0 | 1 | 2 | 0 | 1 |
| Lung/bronchial | |||||||
| Normal | 5 | 4 | 5 | 5 | 5 | 4 | 5 |
| Alveolar macrophage aggregation (1) | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Pigments, alveoli (1) | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Stomach | |||||||
| Normal | 5 | 5 | 5 | 5 | 5 | 5 | 4 |
| Vacuolation, squamous epithelium, limiting ridge (2) | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Ileum | |||||||
| Normal | 5 | 5 | 5 | 5 | 5 | 5 | 3 |
| Dilatation of lymph vessels, Peyer’s patch (2) | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Lymphocyte rich lymph vessels (1) | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Colon | |||||||
| Normal | 5 | 5 | 4 | 5 | 5 | 5 | 5 |
| Lymphocyte rich lymph vessels (1) | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Liver | |||||||
| Normal | 4 | 5 | 5 | 4 | 0 | 0 | 0 |
| Hypertrophy, hepatocellular (2) | 0 | 0 | 0 | 0 | 5** | 0 | 0 |
| Hypertrophy, hepatocellular (3) | 0 | 0 | 0 | 0 | 0 | 5** | 5** |
| Infiltration, mononuclear (1) | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| Infiltration, peribiliary (1) | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Kidney | |||||||
| Normal | 4 | 4 | 4 | 4 | 5 | 4 | 3 |
| Basophilia, tubule (1) | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Cyst (2) | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Dilatation, pelvis (1) | 1 | 0 | 1 | 0 | 0 | 1 | 0 |
| Dilatation, pelvis (2) | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Vacuolation, urothelium (1) | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
BrdU labeling index (LI)
BrdU LI is shown in Table 6. A statistically significant decrease was noted only in the tongue in the PB of the 300 µg/mL group compared to the control group. The change was considered to be incidental because of no dose relationship in the PB groups. No significant changes in BrdU LI in any organ and tissue examined in the study were found both in the TPA and PB treated groups.
Table 6
BrdU labeling indices (%) at GI organs in a 4-week repeated dose study
| Group | Control | TPA | PB | ||||
|---|---|---|---|---|---|---|---|
| Dose level (μg/mL) | 0 | 0.5 | 1.5 | 4.5 | 100 | 300 | 900 |
| Total number of rats | 5 | 5 | 5 | 5 | 5 | 5 | 4a |
| Organ | Mean SD | Mean SD | Mean SD | Mean SD | Mean SD | Mean SD | Mean SD |
| Skin | 11.66 3.21 | 11.60 3.01 | 10.12 3.21 | 8.74 3.35 | 10.62 3.72 | 9.66 2.09 | 10.33 2.15 |
| Liver | 0.88 0.56 | 0.84 0.33 | 0.90 0.72 | 0.72 0.44 | 0.80 0.41 | 0.60 0.32 | 1.10 0.41 |
| Tongue | 19.80 1.97 | 19.10 4.03 | 19.44 2.80 | 20.82 2.16 | 16.68 3.32 | 15.52* 1.89 | 16.60 7.38 |
| Esophagus | 11.96 2.90 | 13.60 1.24 | 12.76 4.02 | 13.88 1.88 | 13.24 2.17 | 11.92 2.80 | 13.25 2.47 |
| Forestomach | 21.96 4.21 | 22.60 2.07 | 20.88 5.92 | 20.72 7.41 | 19.92 4.65 | 22.00 2.67 | 25.25 2.08 |
| Glandular stomach | 7.12 2.46 | 5.08 1.56 | 5.48 0.97 | 7.76 1.18 | 6.08 1.04 | 5.28 1.32 | 4.95 0.38 |
| Duodenum | 36.52 4.77 | 34.68 4.35 | 33.84 1.82 | 38.32 3.61 | 35.40 5.12 | 35.00 3.98 | 33.90 4.82 |
| Jejunum | 43.64 5.45 | 47.28 3.10 | 43.24 2.90 | 45.20 2.92 | 47.36 5.75 | 43.68 3.37 | 41.05 3.86 |
| Ileum | 45.32 2.09 | 42.40 2.29 | 41.12 4.92 | 41.76 6.26 | 40.32 6.42 | 40.24 3.69 | 38.30 4.39 |
| Cecum | 20.00 3.56 | 19.80 2.05 | 18.88 2.75 | 19.20 3.57 | 20.28 3.83 | 17.76 1.39 | 18.90 1.74 |
| Colon | 18.88 3.37 | 18.04 2.71 | 17.80 2.79 | 17.56 1.56 | 17.52 2.16 | 18.52 2.22 | 17.65 3.18 |
| Rectum | 16.92 3.53 | 17.88 2.78 | 20.04 3.36 | 22.16 7.19 | 19.64 1.44 | 19.76 2.67 | 18.10 4.36 |
DISCUSSION
The present study examined cell proliferation activities and histopathological changes in the target and GI organs in rats by oral subacute treatment of tumor promoters, PB and TPA, and provides the first evidence that there is substantially less concern for cell proliferation.
PB or TPA were given to rats orally in drinking water for 4 weeks, and cell proliferation was assessed by histopathology and BrdU immunostaining in the GI organs as well as target organs, liver or skin; however, cell proliferative activity was not found in GI organs. PB and TPA as representative tumor promoters were selected in the present study. PB is a well-known hepatic enzyme inducer and liver tumor promoter as well as a non-genotoxic hepatocarcinogen18. PB activates the constitutive androstane receptor (CAR) which is a xenobiotic-responsible transcription factor belonging to the nuclear receptor gene family, and CAR is essential for PB-induced hepatocyte proliferation and liver tumor development19. TPA is one of the phorbol esters and is also well-known as a potent tumor promoter for skin in mice20 and esophagus in rats21. Topical application of TPA causes epidermal ornithine decarboxylase (ODC) induction and skin inflammation22, and protein kinase C (PKC) activation. PKC is the major receptor for TPA23, and this suggests the involvement of skin tumor promotion by TPA24. It was also reported that activation of the glycolytic pathway25, and epidermal p65/NF-kB signaling is involved in skin carcinogenesis in mouse DMAB/TPA skin tumor model26.
The rationale for dose levels of PB and TPA applied in this study were explained as follows: in the case of PB, a concentration of 500 ppm of PB in drinking water enhanced g-glutamyltranspeptidase positive foci in diethylnitrosamine initiated rats27. The incidence of diethylnitrosamine-initiated hepatocellular carcinoma was enhanced by 250, 500, and 1000 ppm PB but not by 62.5 or 125 ppm PB in drinking water28. In this study, PB was given at concentrations of 100, 300, and 900 µg/mL in drinking water, and thus it was evident the doses of PB were adequate to induce tumor promotion effects on the liver. The application of TPA in skin tumor promotion studies was usually a topical route to the skin. It was reported that increased tumor promotion activity of TPA (as of an expression of activator protein-1, AP-1) was confirmed in the skin and esophagus in AP-1 transgenic mice at a concentration of 0.2 µg/mL in drinking water or 10 µg/mouse by gavage29, and enhancement of esophageal carcinogenesis was observed in N-amyl-N-methylnitorsamine (AMN)-initiated rats at 0.1 µg/mL in drinking water21. In this study, average intakes of TPA were estimated around
60–450 µg/kg/day at concentrations of 0.5–4.5 µg/mL in drinking water. Therefore, dose levels of TPA in this study were considered to be high enough to induce promotion effects in the skin and esophagus.
After 4 weeks of oral treatment, no significant changes in BrdU LI were confirmed in any organ and tissue including skin, liver, and GI organs, both in the TPA and PB treated groups. Histopathological examination revealed no treatment-related findings in any organ/tissue except for hepatocellular hypertrophy in the PB-treated groups, which was considered to be an adaptive change due to hepatic enzyme induction by PB treatment30. In the past, the time-course of hepatocellular proliferation after PB treatment was investigated in rats. Male rats received 50 or 80 mg/kg/day of PB orally by gavage for up to 7 days, and cell proliferation was examined in the liver by BrdU or PCNA immunohistochemistry13,14. PB treatment resulted in the peaks of proliferative activities of hepatocytes on Day 3 and returned to control levels within Day 7 both in the studies. These study results suggested proliferative response in the liver by PB treatment is transient and returned to normal levels during the treatment period. Therefore, no significant changes in BrdU LI in the liver in PB treated group for 28 days in this study are likely to be expected. In TPA-treated groups, there were no notable findings in the target organs of TPA, skin, and esophagus. Even by the different routes of administration, TPA manifests promotion effects on the skin29. In the case of non-TPA type skin tumor promoters such as okadaic acid, a single oral administration of okadaic acid and its related compounds showed dose-dependent increases in cell proliferation of GI organs in rats, and increased BrdU IL also observed in the skin in mice12, and suggested that the okadaic acid class of compounds may exert promoting the potential for GI organs when administered orally. Inhibition of protein phosphatases 1 and 2A is a hypothesized mechanism of tumor promotion by okadaic acid increasing protein phosphorylation and a subsequent expression of cell proliferation genes31. It is not clear whether similar results are obtained by repeated treatment or not. Thus, no studies have directly investigated the exposure effects of high concentrations under subacute conditions in GI organs, and this study provides evidence that substantial cell proliferation concerns are likely to be minimal.
Based on all the above information, the duration of treatment and exposure levels of PB or TPA in this study were judged to be feasible for assessing the cell proliferative activity and tumor promotion effects of PB or TPA on the target organs as well as GI organs. Hakura et al.32,33 reported that BP and the other colon-mutagenic non-carcinogens (CMNCs) induced colon tumors after treatment of dextran sulfate sodium (DSS), a colitis inducer as well as a potent colon tumor promoter, within a short period. In the future, it may be valuable to investigate the effects of the combination treatment of CMNCs and PB or TPA on the colon and the other GI organs to elucidate the effects on carcinogenesis of GI organs caused by accidental oral intake of tumor promoters as contaminants in foods.
Although our study showed little concern about cell proliferation, further investigation is needed to extend this finding to human carcinogenic risk. Because tumor development may be a slow process in humans, a four-week exposure may not be sufficient to detect cell proliferative activity. Furthermore, the effect of carcinogenic agents depends on the status of already carcinomatous cells and cells in the gastrointestinal tract. Differences in species, gender, and genetic background would be taken into account in estimating carcinogenic risk.
CONCLUSIONS
To investigate cell proliferation in target and GI organs of tumor promoters when subacute oral intake of tumor promoters, PB or TPA were given to rats orally in drinking water for 4 weeks, and cell proliferation was assessed by histopathology and BrdU immunostaining in the GI organs as well as target organs, liver or skin. As a result, the present study provides an initial indication that there may be a substantially less concern for cell proliferation. Subacute oral exposure to a sufficient amount of tumor promoters (target organ: liver or skin) as contaminants in foods did not cause cell proliferation in the target and GI organs within our study, and this finding contributes to qualitatively determining the carcinogenic risk of unexpected food contamination of carcinogenic promoters.