INTRODUCTION
Ulcerative colitis is a chronic immune-mediated complex inflammatory bowel disease (IBD) most likely resulting from an interplay between genetic susceptibility and epigenetic events that lead to dysregulation of the immune system associated with inflammation of the rectum, often extending proximally to other segments of the intestine. UC is characterized by symptoms of rectal inflammation: bleeding, tenesmus, and multiple stools daily.
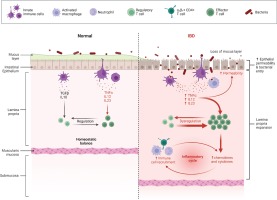
At the cellular and molecular level, the colonocytes (colonic epithelial cells) are key players in the pathogenesis of ulcerative colitis as they display in this case an impaired expression of peroxisome proliferator-activated receptor γ (PPAR-γ) which is a nuclear receptor that downregulates inflammation through nuclear factor kB (NF-kB)1,2. Goblet cells may also play an important role in ulcerative colitis progression as they contribute to the integrity of the mucosal barrier by producing the trefoil factors3,4. A permeable mucosal barrier was often associated with ulcerative colitis mainly due to the ease access of the bacteria through the normally impenetrable inner colon mucus layer5. More, patients with ulcerative colitis usually display dysbiosis characterized by an overall decreased biodiversity, decreased proportion of Firmicutes and increased proportion of Gammaproteobacteria and Enterobacteriaceae 6. Interestingly, it is still not clear whether this dysbiosis is the cause or the effect of the mucosal inflammation as UC associated inflammation may also be triggered by the increased activation and sensitivity of mature dendritic cells7. Also, an increased expression of Toll-like receptors (TLR) that mediate host response via stimulation of NF-kB was also reported8. The inflammatory cascades triggered by NF-kB produce signaling pro-inflammatory cytokines such as tumor necrosis factor α (TNF-α), IL-12 and IL-239,10 which further potentiate lymphocyte activation and proliferation. The antigen presenting cells as well as the T cells activate the innate immune response which will subsequently activate the adaptive immune system9,10. Some studies report UC as a modified T-helper-2 (Th2) disease as an imbalance between regulatory T cells and effector T cells was observed11. The Th2 response activates natural killer T cells (NK) in the colon, which secrete IL-13 triggering epithelial cells apoptosis and tight junctions interruption11 (Figure 1)
Figure 1
Normal versus pathological state of the colonic inflammatory status (Created by BioRender.com)
The diagnosis of UC is not easy to establish, therefor an overall interpretation of several approaches would be advisable. Consequently, the clinical manifestations should be interpreted together with the results of the laboratory tests, lower gastrointestinal endoscopic examination (colonoscopy) and histopathology examination findings. Also, an infectious etiology should be also ruled out.
Macroscopically, the endoscopic findings include loss of vascular markings, granularity and friability of the mucosa, erosions, ulcerations, spontaneous bleeding12,13. Predictor factors of an aggressive course are: young age (<40 years), extensive disease, large and deep ulcers, extra-intestinal symptoms, and an early need for corticoid therapy administration14.
The management of patients with UC includes remission of both clinical and endoscopic features. The choice of medical therapy is based on activity, severity, extent of inflammation and includes oral, topical and/or systemic therapy13. About 15% of UC patients develop an episode of severe colitis and approximately 30% of these patients need colectomy15,16; furthermore, 10% of patients may need surgery in the first year of illness, mostly emergency procedures17. Studies have shown reduction in colectomy rates after infliximab therapy18.
Fournier’s gangrene, described by Jean Alfred Fournier in 1883, is a necrotizing infection caused by multiple organisms, including aerobic/anaerobic species such as Escherichia coli (E. coli) and Bacteroides fragilis rapidly spreading through the superficial/deep fascial layers in the perineal, genital, or perianal regions, causing multiple organ failure/septic shock. The incidence rate has been reported to be approximately 1.6 per 100000 males and the mortality rate around 40% that can be reduced with early recognition/rapid application of adequate treatment protocols including administration of broad-spectrum antibiotics/emergency surgical debridement19. Diabetes mellitus, alcoholism, and immunosuppression are the most important risk factors20. Fournier’s gangrene is a very rare complication of UC reported in four patients to date21-24.
CASE PRESENTATION
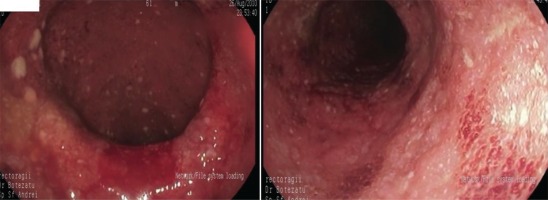
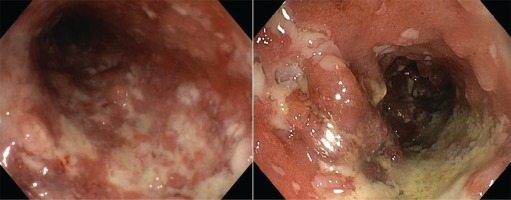
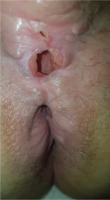
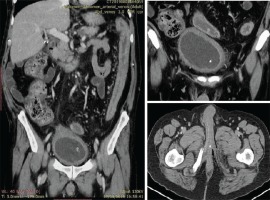
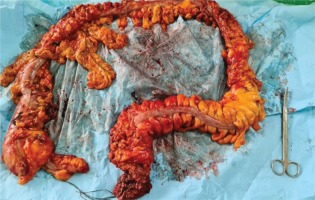
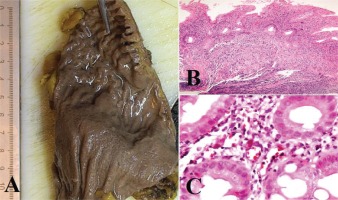
We present the case of a man aged 70 years with UC. Patient’s history revealed posttraumatic urethral stricture with multiple dilatations, multidrug-resistant urinary tract infection (Proteus mirabilis, Klebsiella), Clostridium difficile (C. difficile) infection, and high blood pressure. He was evaluated in the gastroenterology department for diarrhea/bloody stools. Colonoscopy highlighted left-sided colitis, Mayo score 3 (Figure 2), for which oral therapy with Pentasa 3 g/day was initiated. Recurrent C. difficile infections followed. Surveillance colonoscopy after two years highlighted severe left-sided colitis, Mayo score 3 (Figure 3). Immunosuppressing therapy (Azathioprine 2 mg/kg) was added with clinical remission. Four months later he was referred to the Emergency Department with fever, scrotal pain and edema. The diagnosis of Fournier’s gangrene was established. Immediate broad-spectrum intravenous antibiotics were used together with surgical debridement, left orchiectomy and permanent cystostomy. Postoperative outcome was favorable but a complex recto-scrotal fistula developed (Figure 4). Endoscopic evaluation showed persistent severe disease (Mayo score 3). Infliximab 5 mg/ kg daily was added for 8 weeks with cessation of Pentasa. The patient displayed partial clinical response but soon showed secondary loss of response of Infliximab and therapy was switched to Adalimumab. Despite systemic therapy, the patient presented hyponatremia, hypokalemia, systemic inflammatory response (CRP 280 mg/L), severe anemia, which required multiple blood transfusions and 6–8 bloody stools daily. Colonoscopy highlighted severe flare-up of UC (Mayo score 10). Corticoid therapy (Methylprednisolone 32 mg daily) was initiated, with partial clinical response. The patient was referred to the surgery department for proctocolectomy. At the time of admission, he presented anemia, urinary tract infection (MDR Proteus mirabilis), urinary bladder stones and C. difficile infection. Vancomycin was initiated and endoscopic stone extraction was practiced (Figure 5). The patient underwent surgery after C. difficile remission and laparoscopic proctocolectomy/end ileostomyfistulectomy with perineorrhaphy, non-cutting seton had been practiced (Figure 6). Postoperative outcome was initially favorable, but in day 6 he presented a worsening of clinical status, nausea, vomiting, profuse perspiration and leukocytosis. Stool samples highlighted C. difficile ileal infection, therefore Vancomycin therapy was resumed. In postoperative day 9, patient experienced ischemic stroke manifested with altered mental status, mixed aphasia and right hemiparesis, for which he was admitted to the intensive care unit. He was discharged after 19 days with lower right limb motor impairment, C. difficile negative, but persistence of Proteus urinary tract infection. Pathologic findings express large areas of ulceration and granulation tissue with severe inflammation, pseudo-polypoid aspect of colonic mucosa, mild inflammation with intraepithelial neutrophils, with cytomegalovirus inclusions. No dysplasia was evidenced on the specimen (Figure 7).
DISCUSSION
The etiology and pathogenesis of UC is complex and resides from an imbalance between intestinal microbiota and mucosal immunity, resulting excessive local inflammation. Disrupted intestinal microbiota can cause perpetuation of inflammatory response and increasing of harmful bacteria in the intestine25. C. difficile, a gram-positive anaerobic bacterium that causes pseudomembranous colitis, strongly recognized as a complication associated with UC patients, increasing the mortality, risk of hospitalization/surgery26. Risk factors of developing C. difficile infection includes recent use of antibiotics, abdominal surgery, immunosuppressant therapy, gastric acid suppression and IBD27. Previous studies showed that 18–25% of IBD patients experienced recurrence of C. difficile infection within 30 days following treatment28,29; recurrence may be attributed either to endogenous persistence or to contamination by a new strain30. Changes in the terminal ileal epithelium after ileostomy create a colon-like environment, which might explain the frequent development of C. difficile ileitis after a total colectomy with end ileostomy31.
Other enteric infections associated with UC have also been reported as a consequence of disrupted intestinal microbiota, most prevalent being infection with E. coli 2 . Fournier’s gangrene is a polymicrobial necrotizing fasciitis or myonecrosis of the perineal, perianal and genital regions, with a rapid progression and high mortality rate ranging from 15% to 50%32. The most common etiologic bacteria in UC are E. coli followed by Bacteroides and streptococcal species20, while other conditions such as diabetes, alcoholism, and immunosuppression rank as the most important risk factors33. Fournier’s gangrene associated with IBD has been reported to date only in four cases21-24. Prompt diagnosis hemodynamic stabilization, administration of broad-spectrum antibiotics, analgesia and wide debridement, combined with colostomy/cystostomy if needed, are the mainstay for successful therapy24. Perianal fistulous disease (PFD) in patients known with IBD, is more frequently associated with Crohn’s disease, than UC (34% vs 4%, respectively)34,35. Perianal abscess or fistula is a disabling complication that requires surgical intervention. Patients with simple perianal fistula require fistulotomy or fistulectomy; those with complex fistula with risk of sphincter injury may imply usage of non-cutting setons36,37.
Surgery setting in UC (urgent, emergent or elective) depends on disease stage and patient’s status. Patients with extended disease/no-response to medical therapy are candidates to proctocolectomy with ileoanal pouch or end ileostomy. Laparoscopic surgery in experienced hands is a safe, feasible solution for elective surgical treatment conferring advantages of minimally invasive approach such as low intraoperative blood loss and early recovery of the patient associated with shorter hospital stay38. Restoration of continuity is often possible, however, with no guarantee of a good pouch function. More, leaking anastomosis and/ or subsequent pelvic sepsis are difficult to approach in the absence of other standard methods than transanal irrigation, percutaneous or surgical drainage. Chronic pelvic sepsis is associated with poor pouch function and pouch failure with development of persisting presacral sinus39-41. Pelvic sepsis should be aggressively treated in order to avoid the longterm complications.